DolPHIN-2: Final follow-up of pregnant women and babies after 72 weeks

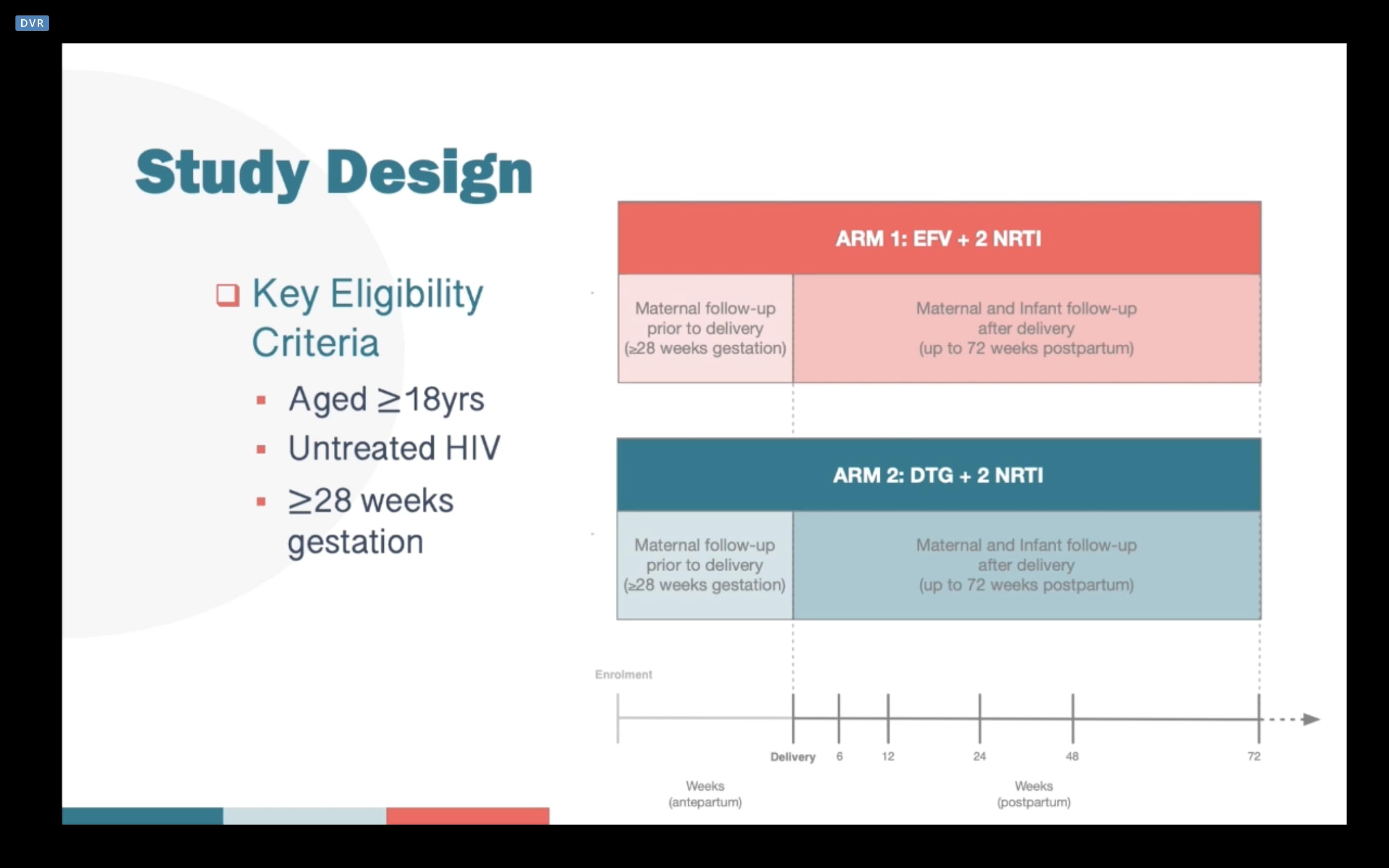
We’ve known for some time that late initiation of HIV treatment in pregnant women can complicate control of the virus and increase the risk of vertical HIV transmission. The DolPHIN-2 study (NCT03249181) looked to analyse the efficacy and safety of dolutegravir (DTG) and efavirenz (EFV) regiments in pregnant women starting treatment in the third trimester.
Preliminary analysis was previously published when the study participants achieved a viral load (VL) of <50 copies/ml at time of delivery. At last week’s Conference on Retroviruses and Opportunistic Infections (CROI 2021) scientists presented the final results of the DolPHIN-2 study – after 72 weeks of observation of the women and their babies.
During the period January to August 2018, 268 pregnant women were selected to receive EFV (133) or DTG (135) of which 250 (EFV 125, DTG 125) were evaluated for efficacy. Viral load (VL) was measured during pregnancy and at 6, 12, 24, 48 and 72 weeks postpartum. The primary endpoints for the study were A) VL <50 copies/ml; and B) frequency of serious adverse events (SAEs) in the woman or infant for safety monitoring.
DTG was found to be significantly superior to EFV regimens at suppressing viral load. On average, it too 4.14 weeks to achieve a VL <50 copies/ml in the DTG group compared 12.14 weeks in the EFV group. There were also fewer therapeutic failures in the DTG group (2.4%) compared to the EFV group (6.4%).
DTG was well tolerated and caused fewer adverse reactions. By week 72, 21.3% of the women and 56.2% of infants had experienced severe adverse events of various kinds. Of these, only 3% were associated with the study drug in the women, and 0% associated with infantile study drug use.
The mean change in maternal weight from the delivery to the 72-week observation point was -1.2KG with slight differences in weight retention by group (DTG 0.7KG versus -1.6KG in the EFV group).
In total, four cases of HIV transmission to the infants were detected. Three during childbirth in the DTG group, one transmission was recorded at 72 weeks in the EFV group – despite the woman having a suppressed viral load (VL <50 copies/ml) and a series of negative tests for the infant.
The results of DolPHIN-2 support the World Health Organisation (WHO)’s clinical guidelines for the use of DTG for the treatment of HIV during pregnancy. Both regimens were safe and well tolerated, however the DTG-based regimen was significantly more effective at controlling infection and maintaining a suppressed viral load during the entire period of breastfeeding.
The study authors concluded by stating that the case of an infection of a child after birth in the EFV group suggests the possibility of continued transmission of the virus through breast-feeding even with a suppressed viral load.



